![]()
RINGNEBULA.COM
|
SCIENCE-3
|
||
|
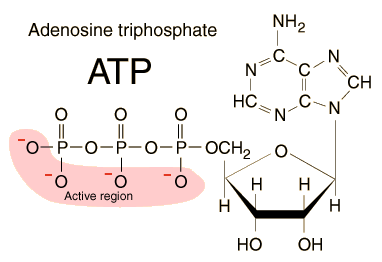
Back of the Envelope ATP Calculations |
|
QUESTION
|
ANSWER
|
||
|
Of all the food calories we ingest, how
|
FOOD TO ATP CALCULATIONS
|
![]()
|
Updated:
April 6, 2019 10:21 AM
|